Short reply to Biotenic’s short thesis on INMB
Introduction
On February 3, 2025, a short thesis on INMB was made public for the first time. That short thesis can be found here:
From a general perspective, the short thesis does not discuss the main thesis on which INmune Bio’s development of XPro for the treatment of Alzheimer’s disease is based.
The author’s assumed cause(s) of AD, XPro’s mechanism of action, or any of the academic papers published on XPro, are not discussed.
The short thesis neither compares biomarker data to what is publicly known or shared by other companies, nor discusses the company’s trial design and enrichment criteria.
In summary, the thesis seems mostly based on an effort to find some weaknesses in the overall story instead of presenting an overall compelling thesis as to why the Phase 2 trial would not yield good results.
I will discuss the arguments from the short thesis below.
1. Argument/statement:
“The company plans to share cognition data for CDR-SB, a commonly used cognition endpoint, as well as their primary endpoint… the EMACC?”
Answer:
The FDA welcomes new rating scales to measure early AD.
EMACC is comprised of validated sub-tests taken from clinically established scales, their inclusion being objective – not subjective – and based on their sensitivity to change in early AD.
INmune made a webinar on EMACC:
INmune Bio webinar: Why EMACC is the Optimal Tool for Measuring Cognitive Change in Early Alzheimer’s Trials
2. Argument:
“They claim this is a validated measure, but a quick search of the clinicaltrials.gov database shows that the only trials mentioning this endpoint are their own.”
Answer:
INmune Bio does not claim it is a validated measure. INmune Bio does consider the validated measures to be blunt. The FDA considers that new rating scales are needed for the assessment of early-stage Alzheimer’s.
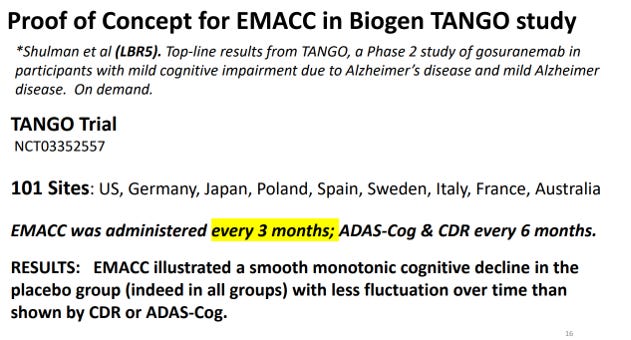
The statement that the only trials mentioning this endpoint are their own is incorrect. Big pharma requested the development of EMACC first. EMACC was first developed in 2017 with the support of Lundbeck Pharma with the goal of finding a replacement for the ADAS-Cog that would measure cognitive changes that occur in Early AD. EMACC was used in Biogen’s TANGO study.
See here for more info on EMACC: https://isctm.org/public_access/Autumn2022/Presentations/Jaeger_Alzheimers_2022_Aut.pdf
Slide excerpt:
For further information, see the INmune Bio webinar ‘Why EMACC is the Optimal Tool for Measuring Cognitive Change in Early Alzheimer’s Trials’:
Time stamp 1h01m.
INMB: “I think you mentioned earlier there are three companies currently running, is that yourself and Biogen, or what are the three companies currently?”
Judith Jaeger / Cognition Metrics: “I'm afraid I have confidentiality agreements with them and I can't share.”
INMB: “Okay was it, is it because I know this Biogen, is there a third company or are there three?”
Judith Jaeger / Cognition Metrics: “There is a third company.”
Biogen’s TANGO study for gosuranemab (BIIB092), an investigational anti-tau antibody that was being evaluated as a potential treatment for Alzheimer’s disease, used it.
3. Argument:
“The Phase 1 data would be a mess because “First of all, the primary endpoint of the study was TEAE rates, which they don’t address anywhere in either disclosure.”
Answer:
As a reminder, XPro is a next-generation TNF inhibitor.
The drug is safe and only causes mild injection-site reactions.
“In January 2021, the company announced results on six patients treated with the 1 mg/kg dose. The drug was safe, with injection site reactions being the main adverse event. After three months, white-matter free water, an exploratory imaging marker of neuroinflammation, was decreased by 5 percent. Participants also registered decreases in multiple inflammatory proteins in the CSF, detected by cytokine assays and proteomics analysis. The changes were sustained in three patients who continued on the drug for a year (press release/webinar). The study was completed in September 2021.”
Source: ALZforum
4. Argument:
“They selectively disclose biomarker data, […]”
Answer:
The biomarker data have been shared extensively both in webinars and in PR’s.
5. Argument:
“[…] and I don’t see any of the cognitive and QOL data, including MMSE (common cognitive endpoint), and the verbal fluency test, which would have read through to the EMACC.”
Answer:
Incorrect, cognition results were not uniformly reported due to the patient pool being comprised of highly heterogenous patients in different stages of disease. Contrary to other companies, INmune Bio did not use the data from its Phase 1 trial to create a hype. But the data exists and can be found, for example, in earlier corporate presentations. Most patients had stable disease, one showed minor progression, one showed minor improvement, and two showed meaningful improvement.
There was a correlation between reduction of neuroinflammation and improvement in cognition.
6. Argument:
“[…] yet they are obfuscating their existing data.”
Answer:
This is incorrect.
7. Argument:
“The data that they did share in the slides also doesn’t make much sense.”
Answer:
I respectfully do not agree. I believe the academic world disagrees as well. See www.thetyp.com for some of my thoughts –to be continued.
8. Argument:
“Very few slides show the data from every dose level, some clearly only show one dose level but don’t even tell you which dose level it is, for some of the biomarkers they pull in an n=36 external database and they only comp it to the high dose. The baselines (which they only have for completers???) are wildly imbalanced, the low dose is entirely mild patients.”
Answer:
The Phase 1 trial was a biomarker-oriented trial.
The trial enrolled a very heterogeneous population.
The comparator was the ADNI database (ADNI | Alzheimer's Disease Neuroimaging Initiative), a well-known database with data on people with Alzheimer’s disease.
Due to a software issue, some of the data of the Phase 1 trial got lost. See INmune Bio webinar September 9, 2021. The patients were not lost. For the MRI data, one of the sites did a software update and did not do the appropriate control, so the quality of that data was lost.
9. Argument:
“If n=3 data at 12 months from a 12 week study is enough to make a 16% claim in the press release, why not comp the other dose levels here too? Surely that data is also interpretable?”
Answer:
As mentioned, some data was uninterpretable due to a software update.
The 1 mg/kg dose is the dose going forward, so it is also the most representative for the upcoming readout. https://clinicaltrials.gov/study/NCT05321498?lead=Inmune%20Bio,%20Inc.&rank=4
10. Argument:
“… what?”
Answer:
The first slide shows data for ptau-217 and p-tau181 from the Phase 1 trial.
The second slide is a longitudinal study showing the correlation of ptau-217 and disease onset.
Not sure what “What?” means otherwise, that would be in support of the short thesis.
11. Argument:
“Where is 0.6 mg/kg?”
Answer:
There is a dose-dependent response.
Here it is.
More DD on INMB, www.thetyp.com.